Enthalpy of Moist Air:
In accordance to Gibb's law, the enthalpy of a combination of perfect gases might be got by the total summation of the enthalpies of the respective constituents. Thus the enthalpy of the moist air h is equivalent to the summing up of the enthalpies of dry air and of the water vapour related with the air. Consequently,
h=ha+ whv ...6.22
Per kg of dry air, here ha is the enthalpy of the dry air part & whv is the enthalpy of the water vapour part. The modification in enthalpy of a perfect gas being referred only as a function of temperature, the enthalpy of the dry air part above a datum of 00C is expressed as following:
ha=Cpa t=1.005 t kJ/kg (=0.24 t Btu/lbm where t is in 0F)
here Cp= 1.005 kJ/kg.K refer for the specific heat of dry air, & t refer for the dry-bulb temperature of air in 0C.
Suppose the reference state enthalpy as zero for saturated liquid at 00C, the enthalpy of water vapour on pt A in the above given Fig may be expressed as following:
hv= hA= Cpw td+ (hfg)d + Cpv (t - td) kJ/kg ...6.24
here Cpw= specific heat of liquid water
td = dew point temperature
(hfg)d = latent heat of vaporization at DPT
Cpv=specific heat of superheated vapour
By taking the specific heat of liquid water like 4.1868 kJ/kg K and that of water vapour is 1.88kJ/kg K, in the range 0 to 600C, we have the following eq.
hv=4.1868 td + (hfg)d + 1.88 (t -td)
For an ideal gas at low pressure, the enthalpy is only a function of temperature. Therefore in given fig the enthalpies at point B & C are also the similar as the enthalpy at A. Hence, enthalpy of water vapour at point A, at DPT of td and DBT of t, might be find out more conveniently through the following two methods:
(1) hA = hC = (hg)t ...6.26
(b) hA = hB= (hfg)0o C+ Cpv ( t - 0) ...6.27
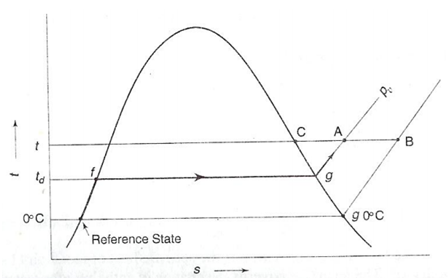
Figure: Evaluation of Enthalpy of Water Vapour Part
By using second expression and by taking the latent heat of vaporization of water at 00C as 2501 k/kgK, we get the empirical expression for the enthalpy of the water vapour part
hv=2501 + 1.88t kJ/kg ...6.28
And joining Eq 6.23 & 6.24, we have the enthalpy of moist air
h =1.005t + ω (2500 + 1.88t) kJ/kg d.a. ...6.29