Modifications to Polymer Chains:
Various factors effect polymer stability. First one, energy-delocalizing aromatic structural groups raise polymer stability through distributing energies of excited states. Further, halogen atoms inside polymers produce free radicals and therefore promote radiation damage.
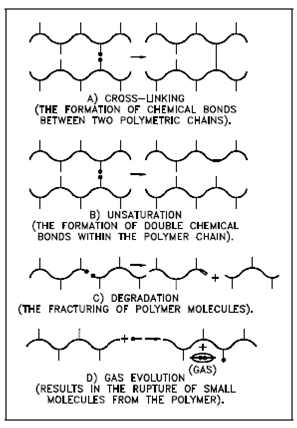
Figure: Modifications to Polymer Chains Due to Irradiation
Substituents on aromatic groups which extend the delocalized bonding network are additionally stabilizers. At last, saturated aliphatics are more radiation resistant than those which are unsaturated; isolated twice bonds are readily excited to radicals or ions.
Organic compounds, in sequence of reducing radiation resistance, alcohols, are aromatics, amines, aliphatics, ketones, esters, and acids. Extension to beta radiation is possibly reasonable. Within tritium gas, therefore, substantial variations in polymer or irradiation surface as compared to bulk could occur. This output from the greater density of tritium (and the widely greater range of the beta in the tritium gas) outside the polymer compared to inside the polymer bulk.
A few direct experiences of polymers along with tritium have been acquired. Viton, Teflon, or Kel-F exposure inside tritium generates the acid TF, noted as SiF4, gas in a glass system. Since of this acid production, tritium + moisture + Teflon in a stainless steel system at pressures of around 1300 atm caused catastrophic stress corrosion cracking of 0.76-mm thick stainless steel tube walls within 16 hours. Substituting deuterium for tritium or removing Teflon or moisture caused no failure. The Radiation damage to Teflon is more severe than to all other thermoplastics. Teflon is thus not recommended within the presence of concentrated tritium streams.