Reactivity:
Amines react like a nucleophiles or bases, as the nitrogen atom has a readily available lone pair of electrons that can participate in bonding described in figure. As a result, amines react with acids to make water soluble salts. This permits the simple separation of amines from other compounds. A crude reaction mixture can be taken out with dilute hydrochloric acid such that any amines present are protonated and dissolve in the aqueous phase as water-soluble salts. The free amine can be recovered through adding sodium hydroxide to the aqueous solution such that the free amine precipitates out like a solid or oil.
Amines will as well react like nucleophiles with an extensive range of electrophiles including alkyl halides, aldehydes, ketones, and acid chlorides. The N-H protons of primary and secondary amines are weakly electrophilic or acidic and will react along with a strong base to form amide anions.
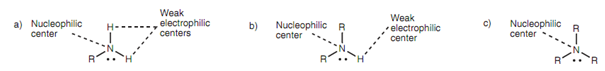
Figure: Nucleophilic and electrophilic centers in (a) primary, (b) secondary, and (c) tertiary amines.
For instance, diisopropylamine (pKa ~40) reacts with butyllithium to provide lithium diisopropylamide (LDA) and butane.