Physical properties:
Amines are polar compounds and intermolecular hydrogen bonding is probable for primary and secondary amines. Hence, primary and secondary amines comprise higher boiling points than alkanes of identical molecular weight. Tertiary amines as well have higher boiling points as compared to the comparable alkanes, but have slightly lower boiling points than comparable primary or secondary amines as they cannot join in intermolecular hydrogen bonding. Though, all amines can participate in hydrogen bonding with protic solvents that means that amines have identical water solubilities to comparable alcohols. Low molecular weight amines are freely miscible with water. Low molecular weight amines have an offensive fish-like odor.
Basicity Amines are weak bases but they are much more basic than alcohols, ethers, or water. As a result, amines work as bases while they are dissolved in water and equilibrium is set up among the ionized form (the ammonium ion) and the unionized form (the free base).
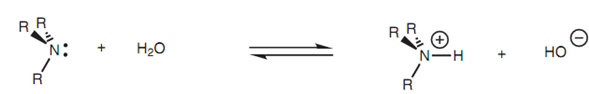
Figure: Acid-base reaction between an amine and water.