Phenols:
Phenols are compounds that have an OH group straight attached to an aromatic ring. Hence, the oxygen is sp3 hybridized and the aryl carbon is sp2 hybridized. Even though phenols share a number of characteristics with alcohols, they have distinct properties and reactions which set them distinct from that functional group.
Phenols can play a part in intermolecular hydrogen bonding that means that they have moderate water solubility and have higher boiling points than as compared to aromatic compounds lacking the phenolic group. Phenols are weakly acidic, and in aqueous solution an equilibrium exists among the phenol and the phenoxide ion. On treatment along with a base, the phenol is completely converted to the phenoxide ion.
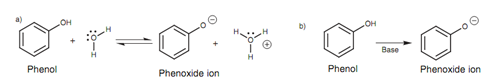
Figure: Acidic reactions of phenol.