Activation of an alcohol:
However, they cannot be employed if water is the solvent because the alkoxide ion would work as a base and abstract a proton from water to regenerate the alcohol. Hence, an alcohol would have to be employed like solvent instead of water.
Nucleophiles that are as well strong bases react with the electrophilic hydrogen of an alcohol rather than the electrophilic carbon. Nucleophilic attack at carbon would need the loss of a hydroxide ion in a nucleophilic substitution reaction. Though, this is not favored because the hydroxide ion is a strong base and a poor leaving group.
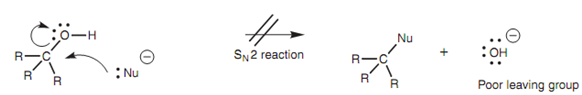
Figure: Nucleophilic substitution of alcohols is not favored.
However, reactions that involve the cleavage of an alcohol's C-O bond are possible if the alcohol is first 'activated' like that the hydroxyl group is transformed into a better leaving group. One technique is to reacts the alcohol within acidic conditions such that the hydroxyl group is protonated before the nucleophile forms its attack. After that cleavage of the C-O bond would be more likely because the leaving group would be a neutral water molecule that is a much better leaving group. On the other hand, the alcohol can be treated with an electrophilic reagent to convert the OH group into a different group (OY) that can then act as a better leaving group. In both of the cases, the alcohol must ?rst work as a nucleophile, with the oxygen atom acting like the nucleophilic center. After that the intermediate formed can reacts more readily like an electrophile at the carbon center.
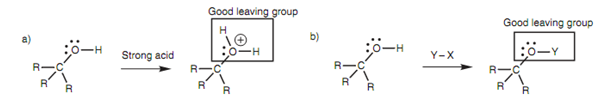
Figure: Activation of an alcohol.