Acid–base properties of alcohols:
The O-H and C-O bonds are both polarized because of the electronegative oxygen, like that oxygen is slightly negative and the carbon and hydrogen atoms are little positive. This means that the oxygen works as a nucleophilic center whereas the hydrogen and the carbon atoms work as weak electrophilic centers.
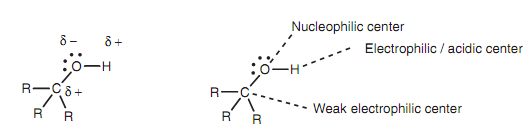
Figure: Bond polarization and nucleophilic and electrophilic centers.
Because of the presence of the nucleophilic oxygen and electrophilic proton, alcohols can act both as weak acids and as weak bases while dissolved in water. Though, the equilibria in both cases are virtually fully weighted to the unionized form.
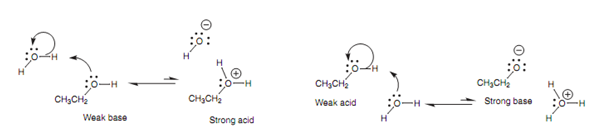
Figure: Acid-base properties of alcohols.