Iron Making
The three raw materials are charged in blast furnace via carrying them to the top of and dumping in the furnace. The furnace principle was enhanced in Central Europe, and the first furnace began operating in 1621 year. In India the first steel plant begins its operation in the early part of twentieth century. The blast furnace is fundamentally a large steel cylinder lined with refractory or heat-resistant bricks and has the height of about a ten- storey building.
The charge mixture is melted in a reaction at 1650oC along with air preheated to about 1100oC and blasted in the furnace thus the term blast furnace via nozzles or tuyeres. Though a number of reactions may take place, fundamentally the reaction of iron oxide along with carbon produces carbon monoxide that in turn reacts along with the iron oxide, decreasing it to iron. Preheating the incoming air is essential sicne the burning coke along doesn't produce sufficiently high temperature for the reactions to happen.
At the bottom of the blast furnace, the molten metal accumulates, whilst the impurities float to the top of the metal. The molten metal is tapped, at intervals of four or five hours into ladle cars. Every ladle car can hold as more as 160 tons of molten iron. The molten metal at this condition has a typical composition of 1.5 percent silicon, 4 percent carbon, 1 percent manganese, 0.04 percent sulphur, and 0.4 percent phosphorous, along with the rest being pure iron. The molten metal is referred to like pig iron. Employ of the world pig comes from the early practice of pouring molten iron into minute sand molds, arranged approximating a litter of small pigs around a major channel. The solidified metal is termed as pig and is employed in making steels and iron. The blast furnace is represented in following figure.
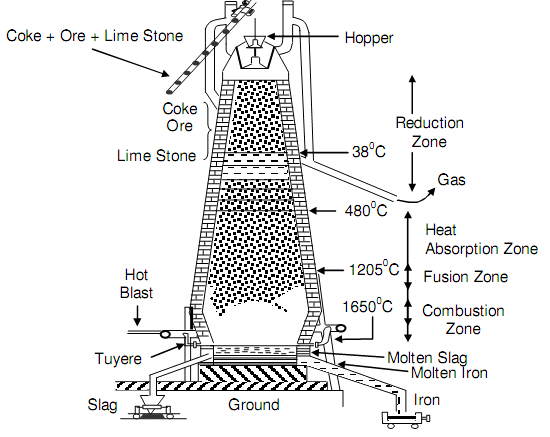
Figure: Blast Furnace