Lambert's Law:
Lambert (1760) and Bouguer independently studied the reduced in the intensity of radiation while it passes by a substance and made the following observations:
i) The amount of monochromatic light absorbed through a substance is proportional to the intensity of the incident light that is. the ratio of the intensity of the transmitted and incident light is constant.
ii) The intensity of the transmitted light decreases exponentially while the thickness of the substance, by that the light is passing, rises linearly.
These observations, known Lambert's law could be translated within a mathematical expression as describe below.
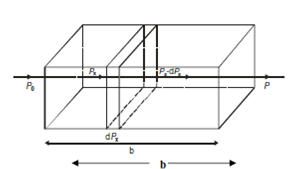
Figure: Illustration for establishing the Lambert's Law
In Figure, if Po represents the radiant power of incident light and P represents the radiant power of transmitted light after passing by a slab of thickness b, consider a little slab of thickness dx, then the change within power (dPx), is proportional to the power of incident light (Px) multiplied by the change in thickness (dx) of the slab through which the light is passed. That is,
dPx ∝ Pxdx
or dPx = k Pxdx
where k is the proportionality constant and the negative sign implies that radiant power decreases with absorption. Eq. could be rearranged to Eq.
dPx/Px = - kdx
Integrating Eq. 2.2 inside the limits of Po to P for intensity and 0 to b for the thickness we get,
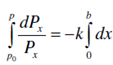
ln = P/PO =-kb
Eq. is a mathematical expression for Bouguer-Lambert law or Lambert's law. Changing this equation to base 10 logarithms and rearranging we get,
log Po/P= k/2.303 b = k 'b
Note that the ratio P/Po has been inverted to remove the negative sign. Lambert's law applies to any homogeneous non-scattering medium, regardless of whether it is gas, liquid, solid, or solution.