Beer's Law:
In 1852, Beer and Bernard independently studied the dependence of intensity of transmitted light on the concentration of the solution. It was found that the relation between intensity of the transmitted light and concentration was exactly the same as found by Lambert for the intensity of the transmitted light and the thickness of the absorbing medium. Through mathematically, a Beer's observations can be expressed as follows.
Consider a monochromatic radiation beam of intensity Px traversing any thickness of solution of a single absorbing substance of concentration c. If c is changed by a small amount dc to c + dc, the change in transmitted power dPx is proportional to the incident intensity Px and dc. We could express it as follows.
dPx ∝ Px dc
dPx = - k "Px dc
where k″ is the proportionality constant and the negative sign implies that radiant power decreases with absorption. This equation could be rearranged to:
dPx/Px = - k " dc
On integration inside the limits of Po to P for intensity and 0 to c for concentration we get
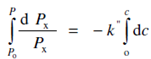
or ln = P/Po =-k"c
or log10 Po/P = (k"/2.303)c
Eq. is a mathematical expression for Beer's law. The Lambert's and Beer's laws are combined and are expressed as per Eq.
log Po/P = abc
In this expression, 'a' is a constant (combining two constants k′, k″ and the numerical factor) and is known as absorptivity (earlier called extinction coefficient) whose value depends on unit of concentration used and is a function of wavelength of the monochromatic light used. A concentration is commonly expressed in terms of grams per dm3 and b in cm. Thus, it has units of c -1 g-1 dm3.