Esters:
There are several different ways where esters can be synthesized. An extremely effective method is to react an acid chloride along with an alcohol in the existence of pyridine. Acid anhydrides as well react with alcohols to provide esters, but are less reactive. Additionally, the reaction is wasteful because half of the acyl content in the acid anhydride is wasted like the leaving group (that is the carboxylate ion). This is not an issue if the acid anhydride is cheap and readily available. For instance, acetic anhydride is helpful for the synthesis of a range of acetate esters.
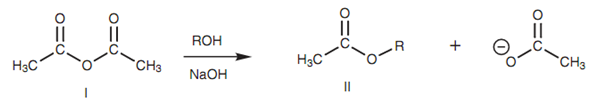
Figure: Synthesis of alkyl ethanoates (II) from acetic anhydride (I).
An extremely common method of synthesizing simple esters is to treat a carboxylic acid with a simple alcohol in the existence of a catalytic amount of mineral acid as shown in figure. The acid catalyst is needed because there are two difficult steps in the reaction mechanism. Very first, the alcohol molecule is not a good nucleophile and thus the carbonyl group has to be activated. After that, the OH group of the carboxylic acid is not a good leaving group and this has to be transformed into a better leaving group.