Rearrangements:
There are 2 rearrangement reactions that can be employed to transform carboxylic acid derivatives into primary amines in which the carbon chain in the product has been shortened through one carbon unit. These are termed as the Hofmann and the Curtius rearrangements. The Hofmann rearrangement includes the treatment of a primary amide with bromine within basic conditions, whereas the Curtius rearrangement includes heating an acyl azide. The end result is similar - a primary amine with loss of the original carbonyl group.
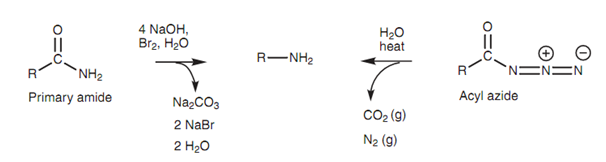
Figure: Hofmann rearrangement (left) and Curtius rearrangement (right).
In both of the reactions, the alkyl group (R) is transferred from the carbonyl group to the nitrogen to make an intermediate isocyanate (O = C = N-R). After that this is hydrolyzed by water to make carbon dioxide and the primary amine. The Curtius rearrangement has the added benefit that nitrogen is lost as a gas which assists to drive the reaction to completion.