Ionization of phthalimide:
Substitution with NH2
Primary alkyl halides and a number of secondary alkyl halides can undergo SN2 nucleophilic substitution along with an azide ion (N3 ) to provide an alkyl azide. After that the azide can be reduced with LiAlH4 to provide a primary amine.
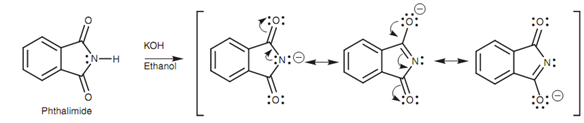
Figure: Ionization of phthalimide.
The entire reaction is equivalent to replacing the halogen atom of the alkyl halide with an NH2 unit. Other method of achieving similar result is the Gabriel synthesis of amines. This includes treating phthalimide with KOH to abstract the N-H proton.
The N-H proton of phthalimide is much more acidic (pKa 9) as compared to the N-H proton of an amide as the anion formed can be stabilized through resonance with both neighboring carbonyl groups. After that the phthalimide ion can be alkylated by treating it with an alkyl halide in a nucleophilic substitution. Consequent hydrolysis releases a primary amine as shown in figure.
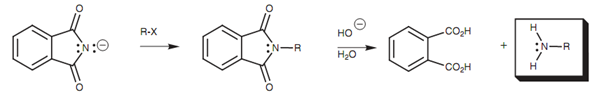
Figure: Gabriel synthesis of primary amines.
A third probable method is to reacts an alkyl halide with ammonia, however this is less satisfactory as over-alkylation is possible. The reaction of an alde-hyde with ammonia via reductive amination is a fourth method of acquiring primary amines.