Arylamines:
The straight introduction of an amino group to an aromatic ring is not probable. Though, nitro groups can be added directly through the electrophilic substitution and then reduced to the amine. The reduction is performed under acidic conditions resultant in an arylaminium ion like product. The free base can be isolated via basifying the solution with sodium hydroxide to precipitate the arylamine.
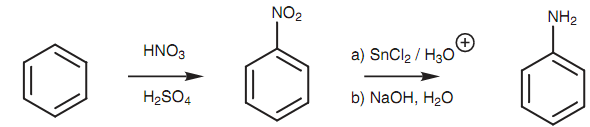
Figure: Introduction of an amine to an aromatic ring.
One time an amino group has been introduced to an aromatic ring, it can be alkylated with an alkyl halide, acylated along with an acid chloride or transformed to a higher amine by reductive animation as explained for an alkylamine.