Alkylation of alkylamines:
It is probable to convert primary and secondary amines to secondary and tertiary amines correspondingly, by alkylation with alkyl halides via the SN2 reaction. Though over-alkylation can be a problem and better techniques of amine synthesis are available.
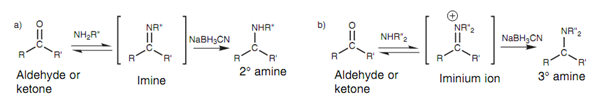
Figure: Reductive amination of an aldehyde or ketone.
Reductive amination is a much more controlled method of adding an additional alkyl group to an alkylamine. Primary and secondary alkylamines may be treated with a ketone or an aldehyde in the existence of a reducing agent termed as sodium cyanoborohydride. The alkylamine reacts along with the carbonyl compound by nucleophilic addition followed through the elimination to provide an imine or an iminium ion that is immediately reduced by sodium cyanoborohydride to give the last amine. Overall, this is the equivalent of adding one additional alkyl group to the amine.