Properties:
Several aromatic compounds have a characteristic aroma and will burn along with a smoky ?ame. They are hydrophobic, nonpolar molecules that will dissolve in organic solvents and are weakly soluble in water. Aromatic molecules may interact with each other by intermolecular bonding through van der Waals interactions. Though, induced dipole interactions are as well possible with alkyl ammonium ions or metal ions in which the positive charge of the cation induces a dipole in the aromatic ring like that the face of the ring is little negative and the edges are little positive. This causes in the cation being sandwiched among the two aromatic rings.
Aromatic compounds are not usually stable and do not react in similar way as alkenes. They prefer to go through reactions in which the stable aromatic ring is retained.
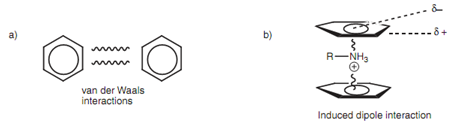
Figure: Intermolecular bonding involving aromatic rings.
The most general type of reaction for aromatic rings is electrophilic substitution, but reduction is as well possible.