Properties:
The meaning of the polar C-X bond is that alkyl halides have a substantial dipole moment. Alkyl halides are weakly soluble in water, but are soluble in organic solvents. They comprise boiling points which are identical to alkanes of comparable molecular weight. The polarity as well means that the carbon is an electrophilic center and the halogen is a nucleophilic center. Halogens are very weak nucleophilic centers and hence, alkyl halides are much more likely to react like electrophiles at the carbon center.
Reactions
The main reactions gone through by alkyl halides are (a) nucleophilic substitution in which an attacking nucleophile replaces the halogen, and (b) elimination in which the alkyl halide loses HX and is transformed to an alkene.
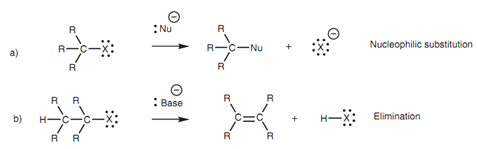
Figure: Reactions of alkyl halides.