Voltammetry:
In voltammetry an electroactive species is consumed (oxidized or reduced only at the surface layer of the indicator electrode in an electrolytic cell. The resulting current, due to the electron transfer process is measured as a function of applied potential. Such electrolysis is carried out under controlled conditions of diffusion (or/and convection). In diffusion layer electrolysis methods only a thin layer of solution immediately adjacent to the electrode undergoes electrolysis. In these methods, the electrical variable is related to the concentration of the bulk, and usually, the time of electrolysis is short that only a negligible fraction of the reactant is electrolysed and the reactant concentration in the bulk solution is not altered (theoretically).
In voltammetry we study the relationship between the current and electrode potential and their applications to chemical analysis. The current potential features are studied commonly, along with the arrangement in which the voltage applied to the cell C is adjusted by means of potentiometer P and the current through the cell is read on a galvanometer G.
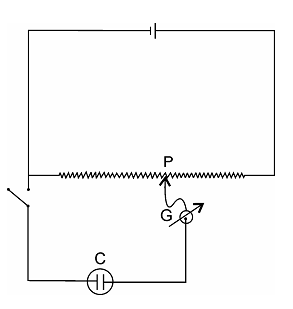
Figure: Circuit diagram for current-potential characteristics
The voltammetric curves are known as current-volts curves or current-potential curve. The shape of the I-E curve depends on the polarization of the indicator electrode, when the other electrode known as the reference electrode remain unpolarized and does not influence the shape of the I-E curve.