Polarography:
Various types of electrodes can be used in voltammetry, but one of them, the dropping mercury electrode (dme), is particularly useful and the corresponding voltammetric method is referred to as polarography.
The use of dme in chemical analysis was originated at the Charles University in Prague, Czechoslovakia in the early 1920s by Heyrovsky who coined the name polarography to designate this technique. The dropping mercury electrode is essentially composed of a capillary connected to mercury reservoir. The bore of the capillary, the length of the capillary, and the head of mercury are adjusted in such a way that a drop is dislodged every 2-6 sec. A platinum wire is immersed in the mercury reservoir, and the dme is coupled with unpolarized electrode.
Polarography consists of electrolysing a solution of an electroactive substance between a dme (cathode) and some reference electrode (anode). The area of the anode is large correspondingly so that it may be regarded as unpolarized and the potential of such electrode remains fairly constant.
The current-potential characteristics can be studied with the type of an apparatus in a simple manner in which the voltage applied to the cell C is adjusted by means of a potentiometer P and the current through the cell is read on a galvanometer G.
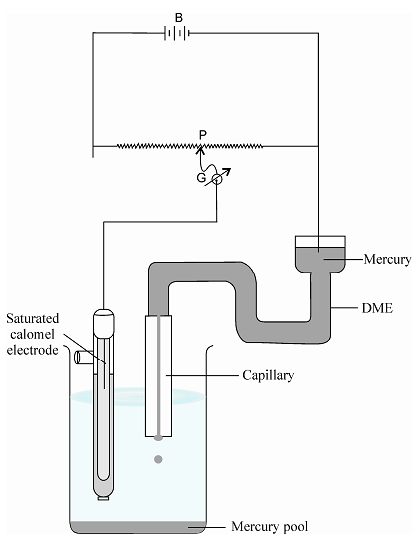
Figure: Manual polarographic circuit
At applying the potential among two electrodes and increasing its value in a stepwise manner the following processes take place. At first only a small current flow - the so called residual current. This continues until the decomposition potential of the reducible ionic species is reached. At this point the following reaction takes place,
Mn+ +ne ↔ M(Hg) (Reducible)
Further a steep rise in current is observed and will continue to rise with increasing potential till the current reaches a limiting value.