Alpha Phase:
The α (alpha) phase is stable at room temperature and has a crystal system characterized by three unequal axes at right angles.
In the alpha phase, the properties of the lattice are different within the X, Y, and Z axes. This is since of the regular recurring state of the atoms is different. Since of this condition, while heated the phase expands within the X and Z directions and shrinks in the Y direction. Below Figure displays what happens to the dimensions (Å = angstrom, one hundred-millionth of a centimeter) of a unit cell of alpha uranium upon being heated.
As displays, cooling and heating of alpha phase uranium could lead to drastic dimensional modification and gross distortions of the metal. Therefore, pure uranium is not used as a fuel, but just in compounds or alloys.
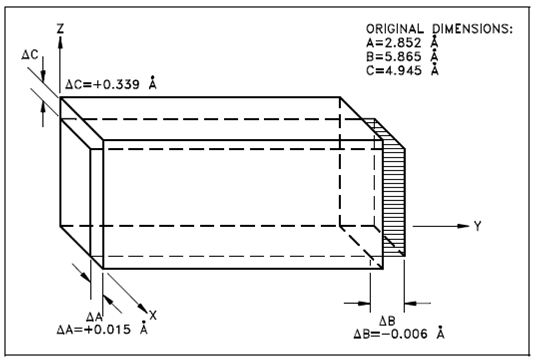
Figure: Change in Alpha Uranium upon Heating from 0 to 300ºC