Determination of 'n' and Reversibility:
It follows from the Eq. (8.13) while a plot of log (i/id - i) is plotted against the corresponding potential of the electrode on the increasing portion of the wave, a straight line will be acquired. A slope of this line will be equal to 0.05915/n from that 'n' could be determined. If 'n' is an integer value we could conclude in which the electrode reaction responsible for this wave is reversible. If a slope of log plot is bigger than the expected or 'n' is not integer value then the wave is irreversible or is a proof of the existence of a few slow steps in the over-all electrode reaction. In like these cases the waves are more drawn-out than reversible waves, or the equation is
Ox + ne- → Re d
A half-wave potential, E1/2 for reversible wave is simply found from a log plot through interpolation to search the value of the potential while the log term is zero.
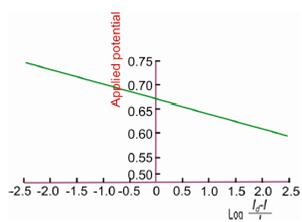
Figure: log plot of Cd(II) in 1M KCl
The other method to determine the reversibility of electrode reaction which is extremely simple and rapid is measuring the potential corresponding to ¼ and ¾ from the polarogram.
At E1/4 for a reversible cathodic wave
E ¼ = E ½ - (0.05915 /n) log (id / 4/id - id / 4)
= E ½ - 0.05915/n log 1/3
and
E ¾ = E ½ - 0.05915/n log 3
so that
E ¾ - E ¼ = - (0.05915/n) log 9 = - 0.0564/n
Therefore, the value of E3/4 - E1/4 is equal to - (56.4/n) mV and for reversible anodic wave it is equivalent to + (56.4/n) mV.