Amperometric Titrations:
According to Ilkovic equation id is proportional to concentration keeping all other factors of the equation constant. Then, if a few of the electroactive material within the solution is removed through interaction along with some other reagent (e.g.: EDTA reagent for Zn2+ determination) the diffusion current will decrease proportionally.
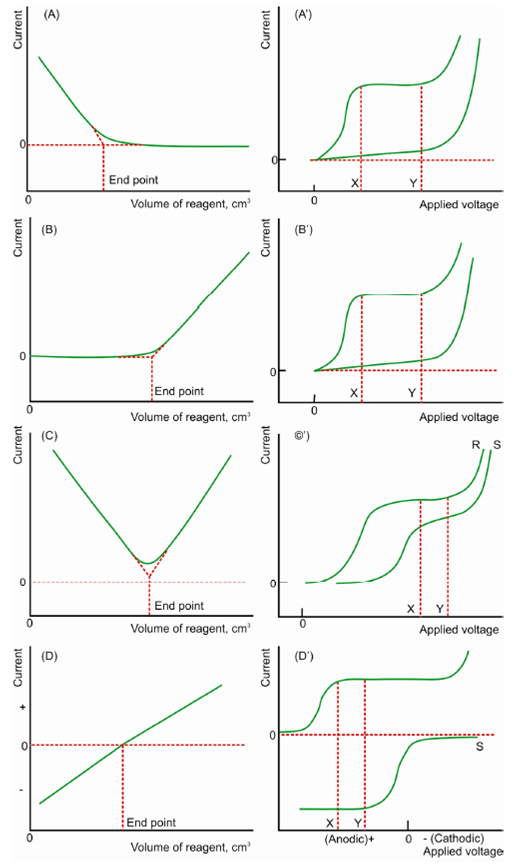
Figure: A-D Common types of curves of amperometric titrations A′-D′ polarograms of individual substances (substance 'S' and reagent 'R')
This is the fundamental principal of amperometric titrations or polarographic titrations. The diffusion current at an appropriate applied voltage is measured as a function of the volume of the titrating solution. The last point is the intersection of two lines providing the change of current before and after the equivalence point.
The voltage applied at the starting of the titration must be selected so in which the diffusion current of the substance to be titrated or of the reagent or of the both, is acquired. Depending on the selection of the applied voltage among DME and reference electrode various kinds of titration curves are acquire as display in Figure.
For every titration the applied voltage is selected to the value among X and Y as shown in figures. A′ to D ′ (S is the substance to be determined and R the reagent).
Figure (A). Electroactive material is being removed from the solution through precipitation or complex formation along with an inactive titrant at which potential applied. e.g. Pb+2 vs Oxalate or Zn2+ vs EDTA.
Figure (B). Substance to be determined does not provide diffusion current but the titrant (reagent) gives current at the applied potential. (e.g: SO4 2- ions titrated along with Pb2+ ions). As long as the substance is available to the titrant, that titrant reacts with substance and current remains zero. After the substance is completely removed through reagent the excess reagent gives its diffusion current within proportional to the excess volume added. Then, the reverse L shaped curve acquire.
Figure(C). Both substance to be determined and the titrating reagent provide diffusion currents at the potential selected (eg. Pb2+ titrated with Cr2O72-).
Figure (D) at the applied potential substance provides anodic current and titrant cathodic current. (eg. I- ion with Hg(NO3)2 )
The titration cell is of Pyrex glass that is a three necked, flat-bottomed flask within that a micro burette, dropping mercury that is an electrode, gas outlet tube for N2 with an additional inlet N2 provision. The cell is linked to a reference electrode in the form of an attachment along with a sintered glass disc separating the titration cell and the RE.