Types Of Polymerisation
While several of bifunctional molecules join in a chain structure, the reaction is known addition polymerisation. This polymerisation does not, though, take place on its own but ought to be initiated, propagated & then terminated. Generally Presence of split H2O2 (2HO) initiates the reaction. Hydrogen peroxide is heated up to split into two free radicals (2HO) that initiates polymerisation. The energy of the chain polymer is lower than that of the independent monomers that compose the chain and therefore the chain grows in length fast after initiation. The reaction is terminated by adding up of trace impurity. The termination of reaction might also occur due to joining of two ends of the chain or because of exhaustion of supply of the monomers. Ethylene (C2H4) polymerising in polyethylene and vinylchloride (C2H3Cl) polymerising into polyvinyl chloride are the instance of addition polymers.
In polymer technology, the developments have led to the understanding that monomers of two different types may join in a single chain in co-polymerisation. For instance, monomers of vinylaecetate and vinylchloride form a chain of copolymers as illustrated in Figure. Vinylacetate molecule with double bond among oxygen & carbon is also illustrated in same figure. This double bond opens into two bonds to join with another opened bond of carbon of vinylchloride.
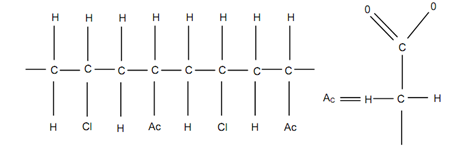
Figure: Co-polymerisation of Vinylchloride (C2H3Cl) and Vinylacetate (OCOCH3)
A copolymer is very much similar to a solid solution in crystalline materials in that its properties are greatly different than those of constituents.
A third type of polymerisation reaction arises while a second non-polymerisation molecule is produced as a byproduct. This reaction is called as condensation polymerization. The usual byproducts of condensation polymerisation are water, HCl or CH3OH. In a typical reaction of polycondensation among hexamethylenediamine and adipic acid long chain nylon molecules are produced with water as a byproduct.