Molecular Structure
The polymers have three different arrangements of molecular chain, therefore resulting in three structures.
In amorphous structure the molecular chain are arranged randomly and bent several times. These chains, as illustrated in Figure (a), cannot move easily past each other due to their entangled nature. Thermosetting plastics contain only this structure and have higher strength because entangled chains needs high stress for their displacement. They also need higher temperature for straightening of chains. Some of thermoplastics also have amorphous structure.
A crystalline structure in polymers needs all the chains to be aligned in one direction as depicted in Figure (b). The geometrical regularity in polythene, illustrated in Figure (c), is the example of crystalline polymer. The symmetry of chains or macromolecules is lost because of attachment of bulky molecule to randomly available bonds in the chain. Polyvinyl acetate illustrated in Figure is an example of amorphous structure.
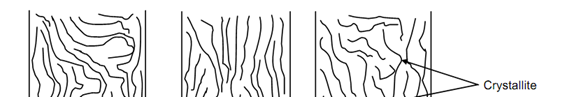
(a) Amorphous (b) Crystalline (c) Mixed
Figure:In certain cases the entire structure might be in groups of crystalline and amorphous macromolecules. Such mixed structure is illustrated in Figure (c). The region where chains are aligned or crystalline is known as crystallite.
In spite of basic chemical nature, the properties of any polymer are affected by its structure. The chain molecules exhibit strength along with their length only if chain molecules generate oils or soft solids as paraffin. The molecular weight of the polymer is controlled by weight fraction of constituents & chain length. Those polymers that have higher molecular weight are tough & more heat resistant. The low density polymers are gummy and soft. The long chain molecules might be cross-linked by introducing such polar groups as hydroxyl, chlorin, carboxyl, flurorin or nitrite. Such cross-linking will enhance secondary inter macromolecular forces and therefore overall strength and rigidity.
Dense crystalline structure provides high melting point (that is sharply defined), high tensile strength & density as compared to amorphous polymer. For the similar reason these materials are brittle. Their flexibility & impact strength might be improved by introducing amorphous characteristic in the structure. Copolymerisation is one of way of reducing symmetry and enhancing flexibility. Copolymer of vinylchloride and vinylacetate called polyvinylacetate illustrated in Figure is the example of improvement in flexibility.
Flexibility of crystalline polymers is also enhanced by addition of some substances called plasticiser. Three general plasticisers are :
1. non-drying vegetable oils,
2. monomeric chemical of high boiling point temperature, and
3. polymeric resinous material of low molecular weight.
These plasticisers occupy location between the molecular chains where the force of attraction among them reduces. This allows them to move relative to each other and therefore flexibility improves. Though, the tensile strength shall reduce.