Functionality
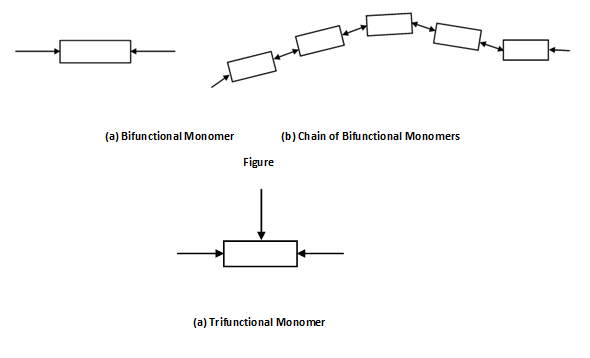
A single unit, like the one illustrated in Figure (a) is called monomer. For a monomer to enter into polymerisation it should have at least two active bonds as illustrated in Figure (b). With these active bonds the monomer might join with other same monomer and form the chain. If the monomer contain more than two active bonds then it may enter into reaction to compose a three dimensional network. The number of active bonds is known the functionality of the monomer. A monomer with two active bonds is bifunctional and may be represented in Figure (a) and its polymerised chain in Figure (b). The ethylene molecule, C2H4, is the example of bifunctional monomer. Phenol molecule (C6H5OH) is the instance of trifunctional monomer. Figure (a) illustrates a trifunctional monomer whereas Figure (b) illustrated resulting polymeric structure. Bifunctional and trifunctional monomers might also join in a single polymer as illustrated in Figure (c).
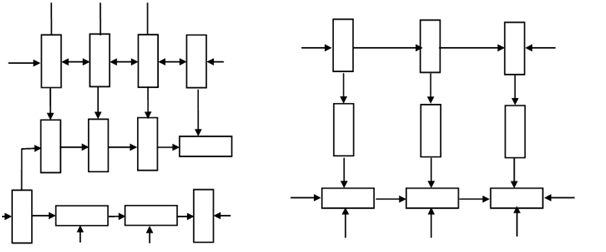
(b) A Polymer of Trifunctional Molecule (c) A Polymer of Bifunctional and Trifunctional