Examples Of Some Common Polymers
Figure illustrated an ethylene monomer with four hydrogen atoms replaced by radicals R1, R2, R3 and R4. Different polymers are achieved by placing such radicals as H, Cl, CH3, COOH3, C6H5, etc. in place of R1, R2, R3 and R4.
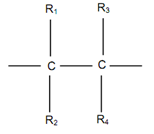
When R1 = R2 = R3 = R4 = H, polyethylene is attained, polyethylene has been illustrated in Figures . It is widely utilized for tubes, sheets and household containers.When R1 = R2 = R3 and R4 = Cl as shown in Figure, the vinyl polymer, known as polyvinyl chloride or PVC, is attained. PVC is largely utilized for electrical insulation & conduit pipes.
.png)
If R1 = R2 = H, R3 = CH3 and R4 = COOH3 a polymer known as polymethyl-methacrylate is attained. This polymer, frequently referred to as PMMA, is illustrated in Figure.
.png)
PMMA is commercially known as, plexiglass , perspecs or lucite. This is strong and hard but is normally utilized for its glass like transparency.
Styrofoam, largely known for its porous structure and thermal insulation is attained when R1 = R2 = H and R3 = R4 = C6H5. It is utilized as insulator in refrigerators and buildings and as sound absorbent.
PTFE or polytetrafluoroethylene, commercially known as Teflon, is chemically resistant to all chemicals except hydrofluoric acid. This polymer is attained by replacing each R by fluorine radical, F. Teflon might retain its hardness up to comparatively high temperature and therefore utilized as coating on razor blades, frying pans and for biomedical applications. It is also utilized as bearing and vacuum ring.