Choice of Argon as Plasma Gas
You may be wondering that in all the three types of plasma sources discussed why is it that argon only is used as the plasma gas? Now let us see the rationale behind the choice of argon. Some of the purpose for this choice is as given below.
i) Argon being an inert gas, does not form stable compounds with analyte elements, therefore there would be lesser chemical interference in the event of atomisation in the plasma.
ii) As its emission spectrum is relatively simple and it is optically transparent in the UV-visible region of the spectrum it does not offer spectral interference in emission spectrometry.
iii) An Argon has a moderately low thermal conductivity, so the heat is retained within the plasma fireball sustaining stable operation at moderate power inputs.
iv) Since the abundance of argon is reasonable (1% in air), it is economical as compared to other noble gases.
v) As the first ionisation energy of argon is quite high (15.75) eV, it has the capacity to atomise, ionise and excite most of the elements of the periodic table which in turn permits the determination of almost all elements that can be excited to emit lines in the UV-visible region.
Nitrogen has been used as plasma gas, either alone or mixed with argon. Therefore, as excited nitrogen emits a complex band spectrum which could cause undesirable spectral interference that has not been hugely adopted. Some investigations have been carried out using helium plasmas. However, because of the high thermal conductivity of the gas and consequent higher power inputs make this also NOT a favourable choice.
Table: Physical properties of plasma gases
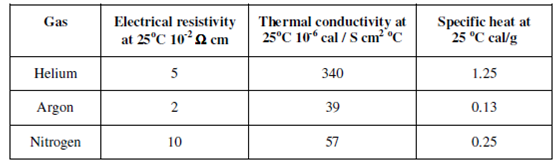
There are only three other gases that have higher ionisation energies as compared to argon and would therefore be suitable for exciting difficult elements such as F, Cl, Br or S. These are fluorine (17.4 eV), neon (21.6 eV) and helium (24.6 eV). In addition, nitrogen has been proposed as a cheap alternative plasma gas. The relevant physical properties of He, Ar and N2 are summarized in table.