Resonance structures for para-nitroaniline:
If an activating substituent is present, able of interacting with the ring via resonance, the opposite keeps true and the para isomer will be a stronger base than as compared to the meta isomer. This is since the crucial resonance structure mentioned above would have a negative charge immediately next to the ammonium ion and this would encompass a stabilizing effect.
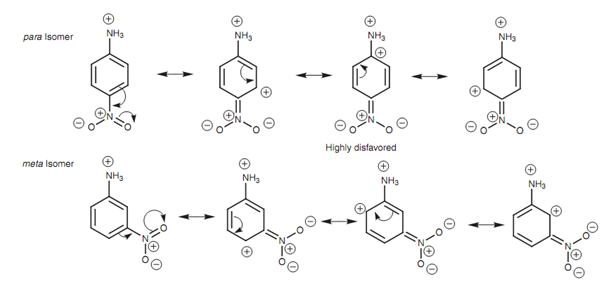
Figure: Resonance structures for para-nitroaniline and meta-nitroaniline.