Inductive effect of an alkyl group:
The amine's basic strength can be calculated by its pKb value (generally 3-4). As much lower the value of pKb, the stronger the base. The pKb for ammonia is 4.74 that compares with pKb values for methylamine, ethylamine, and propylamine of 3.36, 3.25 and 3.33, correspondingly. This illustrates that larger alkyl groups raise base strength. This is an inductive effect whereby the ion is stabilized through dispersing some of the positive charge over the alkyl group. This shifts the equilibrium of the acid base reaction in the direction of the ion, which means that the amine is more basic. The larger the alkyl group, the more major this effect.
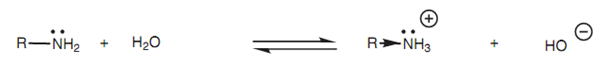
Figure: Inductive effect of an alkyl group on an alkylammonium ion.
Further alkyl substituents must have an even greater inductive effect and one might suppose secondary and tertiary amines to be stronger bases as compared to primary amines. This is not essentially the case and there is no direct relationship among the basicity and the number of alkyl groups linked to nitrogen. The inductive effect of more alkyl groups is counter balanced through a solvation effect.