Photoreceptor transduction
The resting potential of the photoreceptor plasma membrane in the dark is quite low, around 40 mV. The light generates a hyperpolarizing receptor potential, the amplitude of which is associated to the light intensity as shown in figure below:
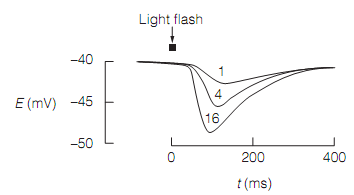
Figure: Cone receptor potentials in response to light flashes of increasing relative intensity (1, 4, and 16).
The hyperpolarization is created by the light-evoked closure of cyclic nucleotide-gated cation channels which are open in the dark. The common, relatively depolarized, condition of the photoreceptor is caused by the flow of dark current, as shown in figure below:
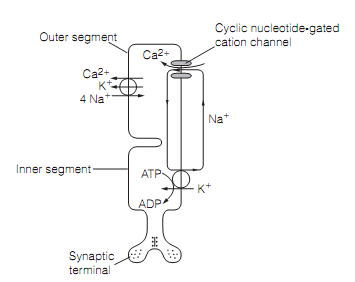
Figure: The ionic basis of the rod photoreceptor dark current.
The cyclic nucleotide-gated cation channel permits Na+ and Ca2+ ions to flow into the outer part in darkness. Na+ ions are actively extruded by the Na+-K+ ATPase in the inner segment. Ca2+ leaves the photoreceptor through a Na+–K+–Ca2+transporter. Whenever light photons strike the outer part a cascade of biochemical events is initiated that result in the closure of the cation channels, decreasing the dark current, and hyperpolarizing the photoreceptor. The transduction procedure in rod cells is well understood. Rhodopsin consists of a G-protein-coupled receptor, opsin, and a prosthetic group, retinal, synthesize by retinol dehydrogenase from retinol (i.e., vitamin A). The retinol cannot be synthesized denovo in mammals and therefore should be supplied in the diet.
In the dark, retinal is present as the 11-cis-isomer. The light causes photo-isomerization to the all-trans isomer. The isomerization takes place within a few picoseconds of the photon being absorbed and triggers a sequence of conformational changes in the rhodopsin to form photoexcited rhodopsin (R*). The Photoexcited rhodopsin couples with a G-protein, transducin (Gt) and swapping of GDP for GTP takes place. The GTP-bound form of the transducin alpha subunit activates a phosphodiesterase (PDE) that catalyzes the hydrolysis of 3’, 5’-cyclic guanosine monophosphate to 5’-GMP. This decreases the concentration of cGMP in the photoreceptor and therefore the cation channels, generally kept open by the cyclic nucleotide, close as shown in figure below:
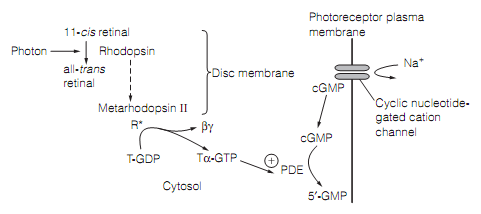
Figure: The role of transducin in photoreceptor transduction. Here, T=transducin; PDE=phosphodiesterase.
This second messenger cascade has a big amplification. A single photon activates around 500 transducin molecules, closes hundreds of cation channels, and blocking the influx of 106 Na+ ions to cause a hyperpolarization of around 1 mV.
Numerous mechanisms act in sequence to terminate the cascade:
- Similar to other G proteins, transducin has an intrinsic GTPase that hydrolyzes the bound GTP to GDP, stopping the activation of PDE.
- The Photoexcited rhodopsin is phosphorylated by rhodopsin kinase, and then ties arrestin that block the binding of transducin.
- Within a few seconds the bond between retinal and opsin in photoexcited rhodopsin spontaneously hydrolyzes and the all-trans retinal diffuses away from the opsin. In high levels most of the rhodopsin exists in this dissociated state in which it is explained as being bleached and the rod said to be saturated. The regeneration of rhodopsin takes place in the dark: retinal isomerase catalyzes the isomerization of the all-trans isomer to the 11-cis isomer which then re-associates with the opsin. This procedure underlies dark adaptation.
The restoration of dark state, additionally, needs the synthesis of cGMP. This is catalyzed by guanylate cyclase. Light adaptation, in which the photoreceptors become less sensitive during light exposure, permits them to respond to levels of illumination which vary by as much as four orders of magnitude as shown in figure. Light-evoked closure of the cation channels decreases Ca2+influx, therefore the Ca2+ concentration in the rod outer segment falls. As Ca2+ generally inhibits the guanylyl cyclase required for cGMP synthesis, this drop in Ca2+ concentration increases the creation of cGMP, offsetting its destruction by the light.
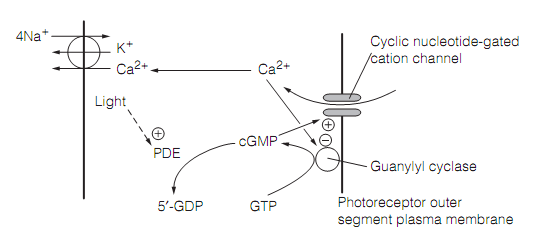
Figure: The role of Ca2+ in photoreceptor light adaptation.