Oxides and oxoacids
P4O6 (4) and P4O10 (5) can be obtained through direct reaction of the elements, the PV compound 'phosphorus pentoxide' being the normal result when phosphorus burns in air. Under carefully controlled circumstances intermediate oxides P4On (n=7, 8, 9) can be complete. The oxides of As and Sb have polymeric structures, and involve a mixed valency compound Sb2O4 with SbIII within pyramidal coordination and octahedral SbV.
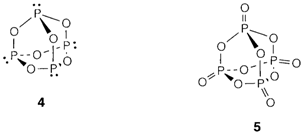
P4O10 is a very powerful dehydrating agent, that is reacting with water to form phosphoric acid H3PO4. This is a weak tribasic acid with consecutive acidity constants exemplifying Pauling's rules: pK1=2.15, pK2=7.20 and pK3= 12.37. Neutral solutions include about equal concentrations of H2PO4-and HPO42-and are extensively used as buffers. A wide range of metal orthophosphates, consisting of ions with each feasible stage of deprotonation, are known. More addition of P4O10 to concentrated phosphoric acid results in the formation polyphosphates with P-OP linkages like in silicates. These linkages are kinetically stable in aqueous solution and are significant in biology. Metaphosphates like KPO3 have infinite chains of corner-sharing octahedra like within the isoelectronic metasilicates like CaSiO3.
The PIII oxoacid phosphorous acid H3PO3 does not comprise the structure P(OH)3 that its formula suggests, except is tetrahedral with a PH bond: HPO(OH)2. So It is diprotic with a identical pK1 to phosphoric acid. The tendency is constant with hypophosphorous acid H2PO(OH). Both the acids are strong reducing agents.
Arsenic acid H3AsO4 is identical to phosphoric acid but is a comparatively strong oxidizing agent. SbV oxo compounds have dissimilar structures and are based on the octahedral [Sb(OH)6]- ion. An Aqueous AsIII and SbIII species are tough to characterize; they are very much more weakly acidic than phosphorous acid and are possibly derived from As(OH)3 and Sb (OH)3. The subsequent salts tend to have polymeric structures, for instance, NaAsO2 with oxygen linked [-As(O-) -O]∞ chains isoelectronic with SeO2.