Other compounds
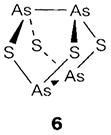
The sulfides of As and Sb are locate in nature. As2S3 and Sb2S3 with the stoichiometries supposed for AsIII and SbIII have polymeric structures. Compounds like As4S4 (6) and P4Sn (n=3-10) are molecules based on P4 or As4 tetrahedra with bridging -S- groups inserted; some of the phosphorus compounds also have terminal P=S groups identical to P=O in 5.
Phosphazines are compounds consisting of repeated -PX2N- units. For instance, the reaction
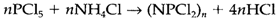
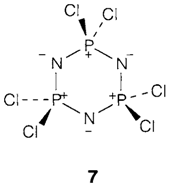
gives chains and rings with a distribution of n values. The (PX2N) unit has similar number of valence electrons like (Me2SiO), which forms silicone polymers. In the valence structure like drawn in 7 P and N carry formal charges, but there is possibly some P=N double bonding.
Binary compounds with metals are usually of low ionic character. Several of those with transition metals comprise the NiAs and related structures and depict metallic properties. A number of compounds appear to consists of polyanionic species (example P24- isoelectronic with S2 2- in Sr2P2, and P73- in Na3P7), even though the bonding is surely not fully ionic.