Hydrides and organic derivatives
The hydrides stibine SbH3 ,phosphine PH3, arsine AsH3 can be prepared through hydrolysis of metal phosphides, or through reduction of molecular compounds like PCl3. The molecules comprise a pyramidal (C3v) structure but with bond angles less than within NH3. They are extremely toxic gases, with reducing thermal stability P>As>Sb. Different from ammonia they are not essential in water. The hydrazine analog diphosphane P2H4 and some other catenated compounds with P-P bonds can be made, even though their stability is low.
Organic derivatives involve alkyl and aryl phosphines like triphenyl phosphine (C6H5)3P. Like with the hydrides these compounds are very much less essential than the subsequent nitrogen compounds in the direction of acceptors like H+, but are good ligands for transition metals in low oxidation states, like they comprise π-acceptor properties. Cyclic polyarsanes like (AsPh)6 (in which Ph is a phenyl group, C2H5) with As-As bonds are readily complete, and with extremely bulky organic groups it is feasible to prepare compounds with E=E double bonds, for instance,
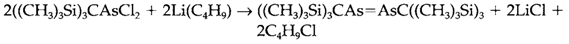
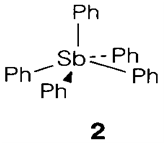
(compare C, Si and Ge; Topic F4). Different with nitrogen, the five-coordinate compounds Ph5E are well-known. The P and As compounds comprise the normal trigonal bipyramidal geometry but Ph5Sb is unpredictably square pyramidal (2).
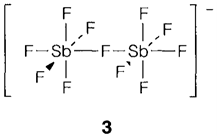
Phosphorus creates the binary compounds P2X4 (through a P-P bond), PX3 and PX5 with all halogens. With As and Sb a comprehensive set of EX3 compounds is identified, but the only EV halides stable below the normal circumstances are AsF5, SbF5 and SbCl5. AsCl5 has been recognized by the UV irradiation of PCl3 in liquid Cl2 but decomposes above -50°C. Several known halides can be obtained through direct reaction of the elements in suitable proportions, but P and F together form just PF5 and the trihalide can be prepared through reacting PCl3 with ZnF2 or HgF2. The molecular substances have the supposed structures, pyramidal (C3v) for EX3 and trigonal bipyramidal (D3h) for EX5. Though, some have a marked tendency to go through halide transfer and within the solid state PCl5 and PBr5 form the ionic structures [PCl4] +[PCl6]- and [PBr4]+Br-, correspondingly. Presumably it is the lattice energy related with an ionic solid that stabilizes these forms. Several halide complexes are known. AsF5 and SbF5 are Lewis acids with a extremely strong affinity for F-, giving [AsF6]- or fluoride bridged species like [Sb2Fn]- (3).
Oxohalides EOX3 create tetrahedral molecules with E=P, but polymeric structures with As and Sb. POCl3 is an significant intermediate in the manufacture of organophosphorus compounds, used, for instance, like insecticides.