Phosphorescence spectrum:
The compounds showing RTP could be separated within two types. The first group involves inorganic salts and oxides of rare earth elements such as, europium, and uranium that phosphoresce naturally. These do not required any sample pre-treatment and the phosphorescence could be measured directly by taking the compounds within a powder holder of the solid sample accessory of the instrument. The other type of compounds, instead, is those that exhibit phosphorescence while adsorbed onto certain supports such as paper, silica, cellulose, etc. The phosphorescence spectrum of salicylic acid adsorbed onto a paper from a solution holding 1 M sodium hydroxide and 1 M sodium iodide is given in Figure.
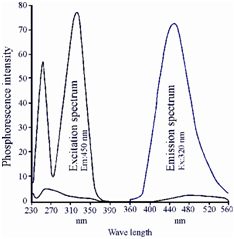
Figure: The phosphorescence spectrum of salicylic acid adsorbed onto a paper from a solution containing 1 M sodium hydroxide and 1 M sodium iodide
This method has been extensively used for the analysis of pesticides, polycyclic aromatic hydrocarbon (PAH), biphenyls, etc. Polycyclic aromatic hydrocarbons are well known group of environmental pollutants that have been found within combustion products of synthetic fuels, cigarette smoke, etc. These are very hazardous, and some of these are established to be carcinogenic in nature. Reliable and cost effective monitoring of PAHs requires rapid screening of samples. The luminescent spectrometry is ideally suited for the reasons because of its sensitivity and simplicity. The phenomenon of quenching is also used for sensitized and quenched phosphorescence at room temperature. It searches extensive applications in polymer chemistry research.