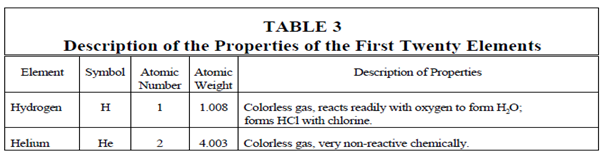
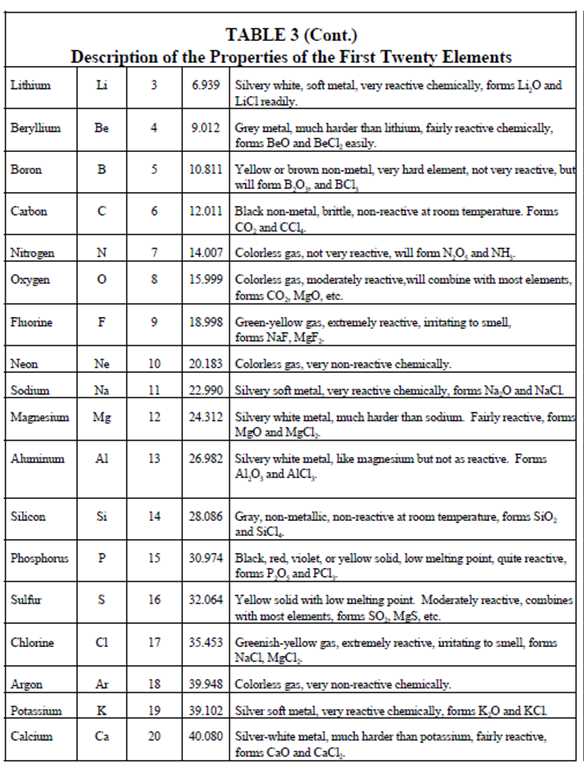
Periodic Table:
Over several years of chemical investigation a scientists have discovered a remarkable characteristic of the elements. The chemical properties of the elements are repeated somewhat regularly if the elements are arranged in the sequence of their atomic numbers. For a lesser extent, a physical property is also repeated periodically. This periodic repetition can be seen within Table. Compare the properties of sodium (Na), lithium (Li), and potassium (K), and also those of magnesium (Mg), beryllium (Be), and calcium (Ca). Within the list of elements display in Table 3 the properties are repeated each eighth element.
A table in that elements along with same chemical properties are grouped together is known as a periodic table. One of the most general versions is display in Figure. Within table, elements are arranged in sequence of increasing atomic number in succeeding rows. Every horizontal row is known as a period. Remember that a few periods are longer than others. Elements along with same chemical properties appear in vertical columns known as groups. Every group is designated through a Roman numeral and a capital letter, excluding the one on the extreme right-hand side and Group 0 (the inert gases). On the bottom of the periodic table are two long rows of elements recognized as the lanthanide series and an actinide series. That is separated from the table primarily to keep it from becoming too wide. In addition, the elements inside each of these two series show same chemical properties.
The number straightly below every element is its atomic number or the number above every element is its atomic weight. In various cases the atomic weights are within parentheses. This denotes which these elements have no stable isotopes; which are, they are radioactive. A value enclosed within parentheses and used for the atomic weight is the atomic mass number of the most stable known isotope, as denoted through the longest half-life.
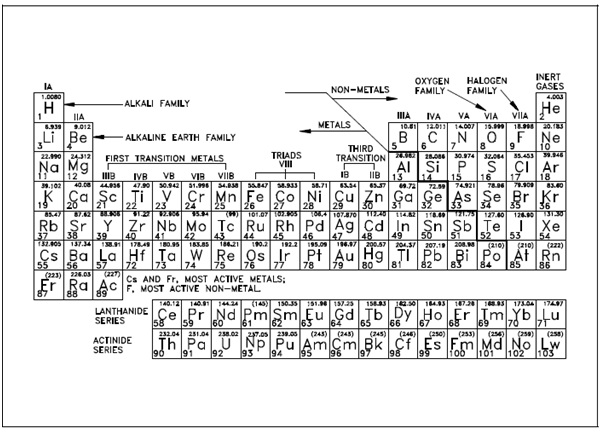
Figure: Periodic Table of the Elements