Block structure
The filling of the table explained above leads to a natural division of the periodic table into blocks as per the outer electron configurations of atoms. Elements of the s block all have configurations (ns)1 or (ns)2. In periods 2 and 3 these are followed instantaneously by the p block with configurations (ns)2(np)x. Lower p block elements are identical as the (n-1)d orbitals are too tightly bound to be chemically significant.
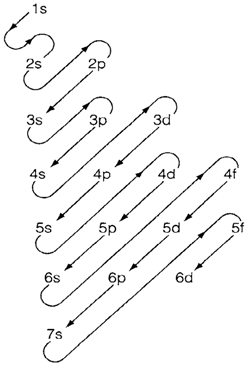
Fig. 1. Showing the order of filling of orbitals in the periodic table.
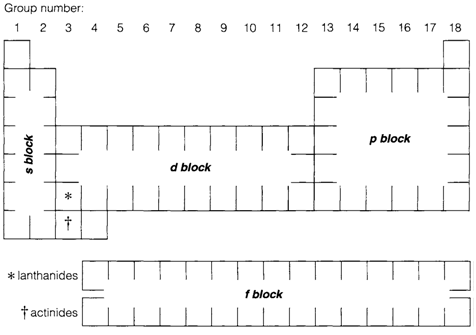
The s and p blocks are jointly known asmain groups. d-block elements of periods 4, 5 and 6 contain ns and (n-1)d outer electrons and are known as transition elements. Their configurations depict some complexities like the s and d orbitals are identical in energy. The f-block elements are termed as the lanthanides (4f) and actinides (5f). For easiness of presentation they are usually displayed as separate blocks below the main table. In the case of the lanthanides, this technique is chemically justified as the elements have very same properties.