Atomic Structure of Electrons:
Based on experimental data, it is called that chemical reactions include just the electrons in atoms. Actually, only a few of the electrons are included. Since chemical properties are periodic, there must also be periodic features about electrons. This feature is the manner in that electrons are arranged within the atom. Electrons are in constant motion around the nucleus. That have both kinetic and potential energy, and their total energy is the sum of the two. A total energy is quantized; that is, there are definite, discrete values of total energy in which atomic electrons could possess. Those energy states could be visualized as spherical shells around the nucleus separated through forbidden areas whereas electrons cannot exist within a stable state. That sort of arrangement is described in below figure.
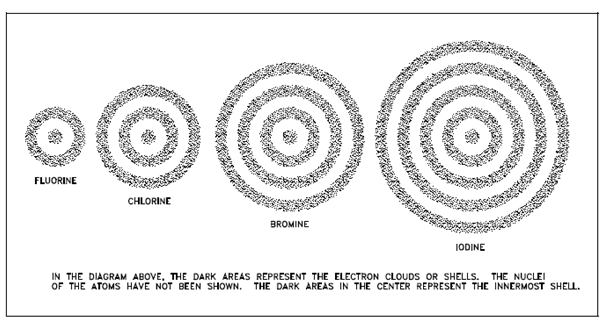
Figure: Electron Shells of Atoms