Galvanic Cell:
The system will be chemically unstable if there is a net conversion of reactants to products in a system, and the reaction will continue until a stable state is attained. That stable state is called as equilibrium.
An active electrochemical cell (oxidation-reduction reaction) is an unstable chemical system. A potential related along with a galvanic cell, for instance, steadily decreases as current flows and the oxidation-reduction reaction proceeds. Eventually, the potential falls to zero; a cell no longer supplies electrical energy, and no additional net reaction takes place. At this point the system is at equilibrium. Within electrochemical cells, a decrease in cell potential caused through the operation of the cell (current flow) is known as polarization.
This modification in cell potential could be determined. Assume the zinc-copper galvanic cell display in Figure. As the reaction takes place, Zn+2 ions (produced through the oxidation of zinc metal) pass into solution. The Cu+2 ions in solution are reduced as the copper metal plates out. Therefore, the concentration of Zn+2 in solution increases and the concentration of Cu+2 decreases according to the following whole reaction.
Zn + Cu+2 → Zn+2 +Cu (1)
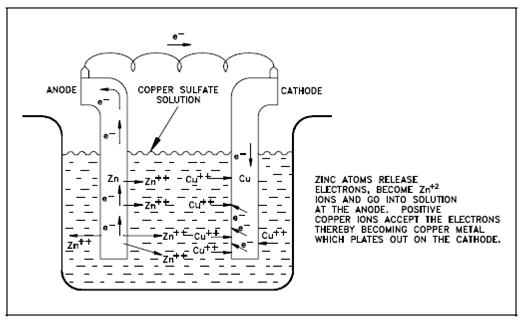
Figure: A Galvanic Cell
Since Zn+2 increases and Cu+2 decreases then the electrical potential decreases. This decrease in cell potential that gives output from changes within concentrations is one form of polarization known as concentration polarization.
Now assume a galvanic cell along with platinum and zinc electrodes, like as that display in Figure. The half-reactions within the cell are given as follows.
Zn→ Zn+2 +2e-
H3O+ + e- → H+H2O (2)
Once more, as the cell operates a cell potential drop. The decrease is partially because of the increase in Zn+2 concentrations and the decrease in H3O+ concentration, other than another category of polarization also occurs within this cell. This second category is related along with the reduction half-reaction.