Electron:
An electron has precisely the same charge quantity as a proton however with opposite polarity. The electrons are far less massive than protons, though. It would take around 2,000 electrons to have similar mass as a single proton. One of the most primitive theories relating to the structure of the atom pictured the electrons embedded in the nucleus such as raisins in a cake. Afterward, the electrons were imagined as orbiting the nucleus, making each and every atom like a miniature star system with the electrons as the planets. The figure is as shown below.
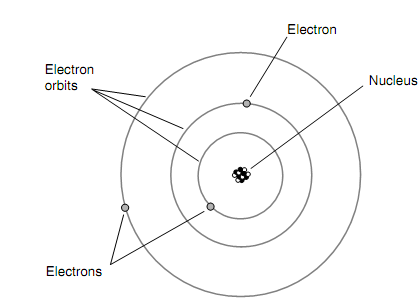
Figure: An early model of the atom, developed around the year 1900.
Still afterward, this view was customized further. In today's model of the atom, the electrons are fast-moving, and they explain patterns too complex that it is impossible to pinpoint any individual particle at any given instant of time. All that can be complete is to say that an electron just as likely will be inside a certain sphere as outside. These spheres are termed as electron shells. And the centers of the shells correspond to the place of the atomic nucleus. The bigger a shell's radius, the more energy the electron has.
Figure shown below is a greatly simplified drawing of what occurs when an electron gains just sufficient energy to "jump" from one shell to another shell presenting more energy.
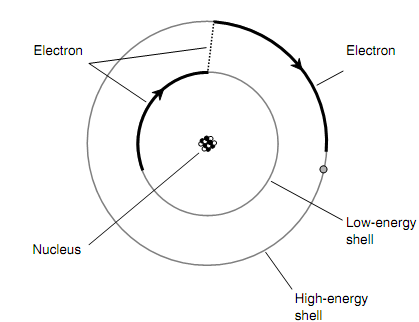
Figure: Electrons exist at defined levels, each level corresponding to a particular, fixed energy state.
Electrons can move instead easily from one atom to the other in some materials. Such substances are electrical conductors. In other substances, it is hard to get electrons to move. These are known as electrical insulators. In any case, though, it is far easier to move electrons than it is to move protons. Electricity almost always outcome, in some way, from the motions of electrons in a material.
Usually, the number of electrons in atom is the same as the number of protons. The negative charges hence exactly cancel out the positive ones, and the atom is electrically neutral. In some conditions, though, there can be an excess or shortage of electrons. The high levels of radiant energy, excessive heat, or the presence of an electrical field can "knock" electrons free from atoms, upsetting the balance.