Supraspinal anti-nociception
Brainstem nuclei that get connections from the spinoreticular tract give rise to descending supraspinal anti-nociceptor pathways as shown in figure.
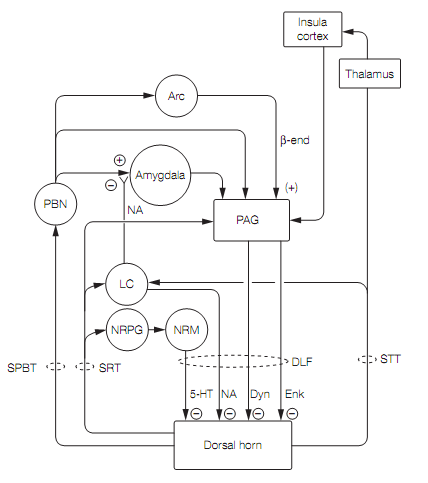
The periaqueductal gray (PAG) surrounds the cerebral aqueduct in the midbrain. Stimulation of the PAG causes a powerful suppression of pain responses and stimulus-induced analgesia. This has been exploited by neurosurgeons that implant chronic electrodes into the PAG of patients with stubborn pain. The PAG exerts its anti-nociception effects by activating brainstem aminergic neurons via inhibitory enkephalinergic neurons acting on inhibitory GABAergic interneurons (inhibiting an inhibition is an excitation). The PAG gets inputs from the limbic system, hypothalamus, amygdala, and cortex (insula), allowing emotional states to modulate anti-nociception.
Neurons in the locus coeruleus (LC) and the nucleus raphe magnus (NRM) in the medulla, which use noradrenaline (norepinephrine) and serotonin respectively, send axons into the dorsal horn. Here the amines reduce transmission from nociceptors into the lamina I dorsal horn cells by:
- Presynaptic inhibition
- Stimulating postsynaptic receptors that open K+ channels causing hyperpolarization of the lamina I cells
- Stimulating lamina II enkephalinergic and GABAergic inhibitory interneurons that hyperpolarize the lamina I cells by opening K+ channels or closing Ca2+ channels
There is debate about whether the effect of serotonergic transmission is pain specific rather than a general effect on all somatosensory input, such as that seen in sleep.
The descending anti-nociceptive pathways appear to be brain analgesia systems. In the event of pain, stress, and/or exercise they produce the natural counterpart of stimulus-induced analgesia termed stress-induced analgesia (SIA). This occurs in individuals who have sustained injuries when they are in threatening or highly arousing situations (e.g., warfare or sport), is rapid in onset and wears off after a few hours. The analgesia can be localized to the injured area or generalized. The selective advantage of SIA is that it allows an individual to continue to function so they can remove themselves from danger. SIA can be endogenous opioid dependent or non-opioid dependent. It seems that opioid analgesia occurs at higher intensity stress than the non-opioid. Opioid SIA requires enkephalinergic neurons in the PAG, while the non-opioid SIA involves the release of endogenous cannabinoids that act on CB1cannabinoid receptors in the PAG.
Brain analgesia is switched on by nociceptor activity in the pain pathways. The spinoreticular tract signals to the nucleus reticularis paragigantocellularis (NRPG) which stimulates the serotonergic cells of the nucleus raphe magnus. The spinothalamic and spinoreticular tracts activate the locus coeruleus noradrenergic neurons. Both these aminergic pathways inhibit the lamina I cells that transmit nociceptor signals. Central noradrenergic pathways also shut down the spinoparabrachial tract by presynaptic inhibition of glutamate release from parabrachial terminals in the amygdala. The mechanism here is unusual. The noradrenaline (norepinephrine) acts at presynaptic α2receptors, liberating the βγsubunits of their associated G protein. The βγ subunits then block vesicle release sites at the active zone of the synapse, curtailing transmitter release.
Electro-acupuncture, which delivers electrical stimulation through needles inserted via skin, relies on stimulation of small diameter afferents from skeletal muscle and joints for its analgesic effect. Low frequency stimulation activates β-endorphin-using neurons in the arcuate nucleus of the hypothalamus. These project to the PAG, activating an enkephalinergic pathway that inhibit dorsal horn cells (DHCs). High frequency stimulation activates the parabrachial nucleus which in turn activates a PAG pathway that uses dynorphin-secreting neurons to inhibit DHCs. Since enkephalin acts on µ and d opioid receptors while dynorphin acts on k opioid receptors, alternating between low and high frequency electro-acupuncture has synergistic effects on the DHCs and results in stronger analgesia than either alone.
Opiate drugs such as morphine and pethidine are full or partial agonists at one or more of the opioid receptors, and generate analgesia by mimicking the actions of the endogenous opioids in the pain-modulating pathways. The effect of opioid analgesics can be rapidly reversed by the competitive antagonists such as naloxone.