Peripheral sensitization
Peripheral sensitization is caused by hyperexcitability of primary nociceptor afferents. At an injury site a wide variety of substances are released including; H+, adenosine, serotonin, histamine, prostaglandins, and numerous peptides such as bradykinin and cytokines. C polymodal nociceptors respond to these with second-messenger-mediated alterations to proteins, such as ion channels, enhancing excitability. For example, prostaglandins phosphorylate sensory terminal Nav channels. This reduces the depolarization needed to activate them and makes the channel open longer, increasing the responsiveness of the cell.
A consequence of nociceptor activation is neurogenic inflammation. Primary nociceptor afferents release both glutamate (which acts as a fast transmitter) and peptides, particularly substance P. The peptides amplify and prolong the effects of glutamate. Glutamate and peptides are co-released from the peripheral terminals as well as the central endings of the nociceptor axon. Hence, stimulus-evoked action potentials are conducted both centrally and, in what is termed an axon reflex, also antidromically along neighboring branches to stimulate secretion from their peripheral terminals as shown in figure. This contributes to the classic signs of inflammation at an injury site since substance P produces vasodilation (heat and redness), increased permeability (swelling), and stimulates mast cells to release histamine that excites itch C fibers.
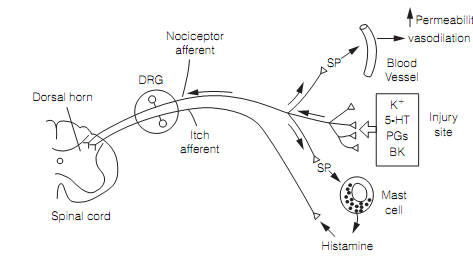
Figure: Neurogenic inflammation produced by axon reflex. The Antidromic action potentials in nociceptor afferent branches stimulate the discharge of substance P (SP) from peripheral terminals. Here, DRG=dorsal root ganglion; 5-HT=serotonin; PGs=prostaglandins; and BK= bradykinin.
This sequence of events can be modified by drugs at a variety of points. For example, prostaglandins lower the threshold of nociceptors to other inflammatory mediators such as serotonin and bradykinin. Aspirin and other non-steroidal anti-inflammatory analgesics (NSAIDs) work by inhibiting cyclooxygenase 2 (COX2), an enzyme involved in prostaglandin synthesis.
The molecular receptors in thermal and polymodal nociceptors which sense noxious heat stimuli (T > 43.C) are capsaicin (vanilloid) receptors (TRPV1). These are ligand-gated Ca2+ channels and so called because they respond to capsaicin, the compound that gives chili peppers their pungency. Activation of TRPV1 triggers transmitter release (glutamate, ATP, substance P) from nociceptor terminals. In peripheral sensitization TRPV1numbers increase, and individual receptors are made more sensitive, by actions of prostaglandin and bradykinin. Attempts to develop capsaicin receptor antagonists as anal-gesics are being thwarted by the involvement of TRPVs in core temperature regulation; TRPV antagonists cause hyperthermia.