Chiral Chromatography:
Quite often only one enantiomer possesses the desired therapeutic activity whereas the other may be inactive or even harmful. The separation of enantiomers by HPLC using chiral stationary phase (CSP) is based on the formation of transient diastereoisomeric compounds between the enantiomorphs of the solute and the chiral selector which is an integral part of the stationary phase. A difference in stability among these complexes results in difference in their retention times, the enantiomer forming the less stable complex being eluted first.
A large number of chiral phases are commercially available. All of these are coated on silica gel support. A coating itself is a polymeric material to which an optically active isomer is bonded. For instance, the l form of the amino acid, proline has been bonded to polystyrene-p-divinylbenzene, and a cross linked copolymer to give an optically active stationary phase for the separation of racemic mixtures of amino acids. In this case, Cu2+ ions are introduced into the solution of the analyte enantiomers to be separated whereby a ternary complex, as shown in Figure. is formed between the stationary phase, amino acid anion and Cu2+. The formation constant for this complex differs for d and l forms of the analyte amino acid; therefore, making their separation possible.
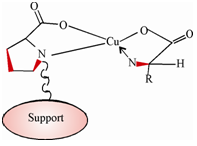
Figure: Illustration of a ternary complex formed between an L-proline bonded phase, an analyte amino acid and a Cu2+ ion