Positive oxidation states
Reaction along with strong oxidizing agents provides the O+2 ion, that has a stronger and shorter bond than O2:
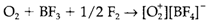
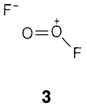
Fluorides comprise F2O and F2O2. The latter has a significantly shorter O-O bond than in peroxides, a fact which may indicate some contribution of ionic valence structures like (3), that permit a degree of multiple bonding. All compounds in positive oxidation states are very powerfully oxidizing. Compounds with heavier halogens are generally reffered as halogen oxides.