Peroxides and superoxides
By Adding one or two electrons to dioxygen produces the superoxide O-2and O2-2 peroxide ions. Like the added electrons take place the π antibonding orbital the bond becomes increasingly longer and weaker. Rather than simple oxides M2O the superoxides MO2 are the general results of reacting the heavier alkali metals with oxygen; peroxides M2O2 are also created. This may be described by lattice energy arguments. Along With most metal ions, the higher lattice energy acquired by the O2- forces the disproportionation of the larger O-2and O2-2 ions. Though, with large, lowcharged cations, the lattice energy achieve is not sufficient to cause disproportionation. The peroxide ion may also be stabilized within peroxo complexes, in which it acts like a ligand to transition metals, like in [CrV(O2)4]3-.
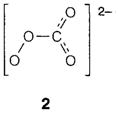
The most simple covalent peroxide is hydrogen peroxide H2O2, which is generally come across in aqueous solution. Even though kinetically fairly stable, it can work like either an oxidizing agent (giving H2O) or like a reducing agent (giving O2), and several transition metal ions catalyze its decomposition. Organic peroxides (R2O2) and peroxoacids (example the percarbonate ion, 2) consist of the quite weak peroxo O-O linkage. Some covalent peroxides can be dangerously and unpredictably explosive.