Extraction of the elements
Very few elements take place naturally in uncombined create. Most of them are found in compounds where they are in positive or (less often) negative oxidation states (example TiIV, ZnII and Cl-I in TiO2, ZnS and NaCl, correspondingly). So, the extraction of these elements requires redox chemistry, by using suitable reducing or oxidizing agents. Thermodynamic considerations are very significant .
Iron is formed in greater quantities than any other metal, by the reduction of Fe2O3 with carbon (coke). The entire reaction approximates to
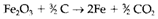
At the 25°C, ΔGΘ for this reaction is +151 kJ mol-1 so that it is not thermodynamically possible at room temperature. Though, it is strongly endothermic (ΔHΘ=+234 kJ mol-1) and so by Le Chatelier's law the equilibrium is shifted in favor of the results at higher temperatures. In a blast furnace it occurs above 1000°C, heat being presented from the combustion of carbon in air, that is blown through the reaction mixture.
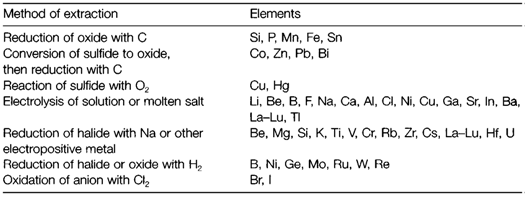
Carbon is a suitable and cheap reducing agent for metal oxides but for several elements it cannot be employed. With some highly electropositive metals (like Al) the oxide is too stable (that is its is too negative) and the temperature needed for reduction by carbon is too high to be economically or technically viable. A number of elements (example Ti) react with carbon to form a carbide. In these examples other redox processes are essential. The Table 1 summarizes the common methods.
Hydrogen can be employed to reduce oxides or halides or a very strongly reducing metal like sodium or calcium to reduce halides.
In electrolysis a redox process with positive ΔG is induced by providing electrical energy. Reduction occurs at the cathode (the negative electrode, that provides electrons) and oxidation at the anode (the positive electrode).
For instance, electrolysis of molten NaCl gives elemental Na at the cathode and Cl2 at the anode. Many extremely electropositive elements (example Na, Ca, Al) and a few very electronegative ones (F, Cl) are get by this method.
Table 1. Extraction of elements from their compounds