Balancing redox reactions
The changes in oxidation state must balance so that the totals on the two sides are similar, in any complete redox reaction. Problems can arise with ions in solution, like the ionic charges may not be similar as the oxidation states. Refer the unbalanced redox reaction in acidified aqueous solution:
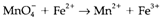
It is very easy to balance the redox changes by first dividing this into two half reactions, one involving oxidation and the other reduction. The oxidation step is
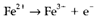 (3)
(3)
with electrons (e-) being separated. The conversion of 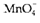 to Mn2+ involves a transform of oxidation state from MnVII
to Mn2+ involves a transform of oxidation state from MnVII
to MnII and so is a reduction requiring five electrons. To balance half reaction
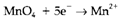
four oxygen atoms are needed on the right-hand side, that (in aqueous solution) will be in the form of H2O. The reaction
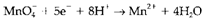 (4)
(4)
is then finished by balancing hydrogen with 8H+ on the left-hand side, like this reaction occurs in acid. The entire redox reaction is now written by combining the two half reactions in such type of a way that the free electrons are removed. This needs 5 moles of Equation 3 to every 1 mole of Equation 4, giving
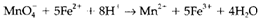
In alkaline solution it is more suitable to use OH- rather than H+. The other species exist may also be dissimilar from those in acid, as several metal cations form insoluble hydroxides or even oxoanions . As an instance, refer the reaction of aluminum metal with water to form [AlIII(OH)4]- and H2. The balanced half reactions are
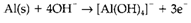
and
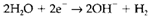
which may be combined in the suitable proportions (two to three) to give
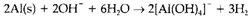
A specific benefit of the half-reaction approach is that it leads naturally to the discussion of the thermodynamics of redox reactions in terms of electrode potentials.