Osmotic equilibrium:
It is probable to derive, from purely thermodynamic considerations, an expression for osmotic pressure within terms of measurable solution parameters. At any constant temperature and pressure of one atmosphere, transfer of solvent within solution occurs since the molar free energy of the pure solvent, μ0, (standard chemical potential of the solvent) is greater than the partial molar free energy of the solvent in the solution μi. To bring equilibrium among the two and therefore, stop osmosis, it is essential to increase the value of μi through applying higher external pressure on the solution. If this rise in free energy is Δμi, then the condition for osmotic equilibrium must be
µi0 = μi + Δμi
and hence
µi0 - μi = Δμi
The chemical potential (μi) of elements, i, in a solution is defined in terms of Gibbs free energy (G) through the relation
dG = SdT + VdP +∑i μi dNi
where, S is the entropy, T is the absolute temperature, V is the volume , P is the pressure and Ni is the number of moles of component, i.
From Eq.,
μI = (∂G/ ∂Ni )T,P,N
and
V = (∂G/ ∂P)T,
where, N represents the entire set of N's and Nj represents all N's except Ni..
Differentiating Eq. with respect to Ni
(∂ 2G/∂ Ni ∂P)T, N = (∂μi /∂P)T,N = (∂V/∂Ni )T,P,N = 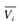
where, 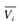 is the partial molar volume of component, i.
is the partial molar volume of component, i.
The thermodynamic activity, ai of elements, i is associated to its chemical potential μi through the relation
μi0 = μi * + RT ln ai
where, R is a gas constant.