Computing the activity of water:
For computing the activity of water within the solution, aw, the vapour pressure data are needed. Often aw is calculated from the relation
aw = Pw / P*w
while, Pw is the vapour pressure of water in equilibrium with the solution at a specified temperature, while Pw* is that of pure water at the same temperature.
Here If the solute is volatile, the partial vapour pressure of water must be used for Pw.
Substituting Eq. 2 in Eq. 3, we get
Π Vw = RT ln ( Pw / P*w)
Eq. can be reduced to a simpler form from the special case of dilute solution of binary electrolyte obeying Raoult's law. For such solution

where, N1 and N2 denote the mole fraction of solvent and solute, respectively. Eq. can be written in terms of solute, the mole fraction as follows:

If ln ( 1- N2 ) be expanded in series, then for dilute solutions all terms beyond the first can be neglected, and ln (1-N2) becomes - N2 which is equal to n2/n1 where, n2 is the number of moles of solute in n1 moles of solvent. Therefore,
Π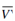 w = RT n2/n1
w = RT n2/n1
and Π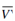 w n1 = n2 RT
w n1 = n2 RT
But w n1 is the total volume of solvent containing n2 moles of solute, which for dilute solutions is essentially the volume of the solution. as a result,
ΠV = n2 RT
or alternately
Π = cRT
where, c is the molarity of the solution. Eq. is well known as van't Hoff's equation for ideal solutions. For electrolytes that ionise in solvent/ water, the observed osmotic pressure is more than what is predicted from molar concentrations data as the osmotic pressure is a colligative property of the solution which depends on the number of species within solution. Accordingly, a correction term called as van't Hoff factor is introduced in Eq. as follows.
Π = icRT
A van't Hoff factor is around equivalent to number of ions produced during ionization per molecule of the electrolyte.