Characteristics of Atomic Spectrum:
You would recall from Blocks 1 and 2 on molecular spectroscopic methods in which the molecular spectra are generally wide. These consist of a number of closely spaced lines constituting what is known as a band spectrum. The band nature of the spectrum is because of a number of factors such as, the quantisation of the rotational and vibrational motions of the molecules along within the quantisation of electronic energy levels. Further the width of the spectrum is also dependent on a few of the instrumental parameters.
In compare to the above, an atomic spectrum consists of a number of extremely sharp lines, features of the atomic species. It denotes that these provides rise to line spectra. The atomic spectra are commonly much sharper because within atomic systems, the rotational and the vibrational motion are not quantised and the transitions are observed amongst the electronic energy levels of the absorbing species. A classic atomic spectrum is shown in Figure.
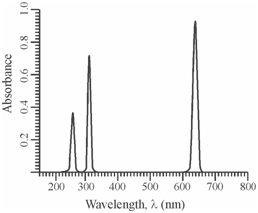
Figure: Representation of a typical atomic spectrum