Origin of UV-VIS Spectrum:
The absorption of radiation in the UV-VIS region of the spectrum is dependent on the electronic structure of the absorbing species such as, atoms, molecules, ions or complexes. The absorption spectrum of atomic species consists of a number of narrow lines which arise as a consequence of the transition amongst the atomic energy levels. You would also recall which in molecules; vibrational the electronic, as well as the rotational energies are quantised. A given electronic energy level has a number of vibrational energy levels in it and each of the vibrational energy level has a number of rotational energy levels in it. While a photon of a given wavelength interacts along with the molecule it might cause a transition amongst the electronic energy levels if its energy matches along with the difference within the energies of these levels.
In the course of such transitions, for the sample within gaseous or vapour phase, the spectrum consists of a number of closely spaced lines (Figure (a)), constituting what is called a band spectrum. Therefore, in the solution phase, the absorbing species are surrounded through solvent molecules and undergo constant collisions along with them. These collisions and the interactions between the absorbing species and the solvent molecules cause the energies of the quantum states to spread out. As a result, the sample absorbs photons spread over a range of wavelength. Thereby, the spectrum acquires the shape of a smooth and continuous absorption peak in the solution phase. A classic UV-VIS spectrum in the solution phase is depicted in Figure (b).
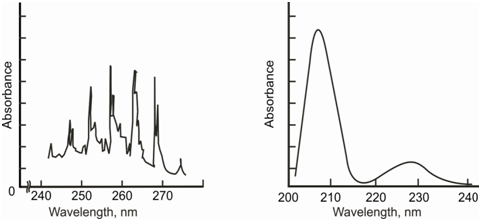
Figure: Representative UV spectrum: a) in vapour phase and b) in solution phase